At Sophysa, we currently have around 230 employees at our various sites in France, Belgium, and the United States. Our people are our biggest asset, and we value every single one of them very highly indeed. With over 75 different professions all under one roof, we have taken every opportunity to help our people develop personally and professionally. All of our employees see a position at Sophysa as a worthwhile career choice, and not just a job.
Sophysa’s growth is driven by our team and their skills. This means we’re continually expanding, thanks to an average increase in the workforce of 9% each year since 2015, and an internal promotion system for career development.
Every year, we invest more and more in professional training both internally and externally. We want our people to develop new skills and stay in touch with developments in our field.
Joining us at Sophysa is your opportunity to take up a technical position and be recognized for your skills and innovative, dynamic contribution to one of the most respected names in medical advancement.
If you think you have what it takes to be a part of our team, we’d love to hear from you. Take a look at some of our trades through the detailed portraits below, and if you think you’d fit in, come and join us. Contact the HR department at Sophysa on +33(0)1 69 35 35 00 for more information.

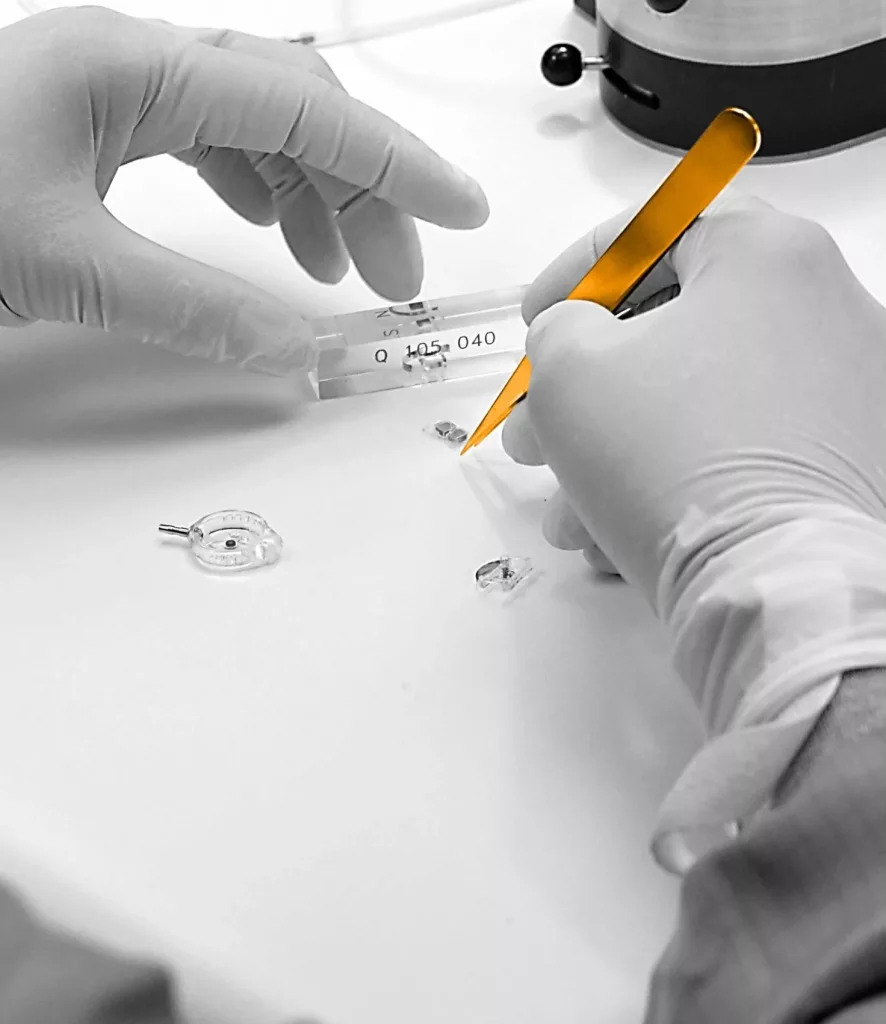
Here, Sophysa’s own people tell you all about their roles within the company, what they do, and how they contribute to the business.
Methods and Industrialization Engineer
The Design Office designs a product. Our role is then to find the solutions to be able to manufacture the products while respecting the requirements of quality and the constraints of production.
My job is organized around three main missions which are:
• The definition of the means of production for new products
• Improvement of existing processes
• Production support
All of these processes have a strong impact on the quality of the product, so they must be validated in connection with the quality/validation team to ensure compliance with product requirements and reproducibility. Each product is identical even if it is made by different operators.
Valve Manufacturing Operator
The work of the Valve Manufacturing Operator breaks down in ½ days, either morning or afternoon.
Each manufacturing operator follows a defined manufacturing process.
It is first necessary to follow the protocol of entry into a clean room, where a full specialist outfit is put on, and the operator then decontaminates their workstation.
The main operations for the manufacture of a valve are:
In parallel, it is necessary to make numerous tests to validate the operations carried out.


Project manager at the Design Office
The Design Office’s role is to develop new products and change the design to improve its performance. Each project manager manages a range of products.
The project manager ensures the coordination of projects by supervising the project group on the three phases which make up a development cycle. These are:
Project Managers also coordinate all the activities of the Design Office as well as providing technical support for the sales and regulatory activities.
Regulatory Affairs Officer
The role of the Regulatory Affairs Officer is to ensure every part of the process and every product complies with all current international regulations. This includes compiling the technical documentation and creating or adapting it to the large target markets, and ensuring it complies with regulations in the individual countries. It manages product registrations in different countries according to these regulations.
Meanwhile, the Regulatory Affairs Manager conducts Materials vigilance when there is a report on any Sophysa product made by a user, usually a healthcare professional such as a neurosurgeon. That is, the issue is reported to the appropriate authorities in the country in which the incident took place and, depending on the case, the incident is then reported in other countries.
The Regulatory Affairs Officer also contributes to Internal Audits.

Sophysa is committed to gender equality
Sophysa is fully committed to guaranteeing and maintaining professional equality between women and men, notably through recruitment, training and remuneration. No form of discrimination is practiced during our recruitment process.
We are fully aware that recruitment is a major issue in professional equality. Therefore, at Sophysa, we guarantee that all applications will be processed with the aim of promoting equality and equity. Sophysa respects the legal obligations concerning this principle of equity and its publication index in accordance with the texts in force.
Gender equality index
In accordance with the law on the freedom to choose one’s professional future, companies are required to calculate and publish a “gender equality index”.
The results obtained over the past three years demonstrate the effectiveness of our commitments, although there is still room for improvement.
The company’s results for the year 2021 were as follows:
-> 99/100 if the 5 criteria are taken into account (for companies with more than 250 employees)
-> 99/100 if the 4 criteria are taken into account (case of Sophysa in compliance with the legal obligations of the company).
The results of the company for the year 2022 are the following:
-> 97/100 if the 5 criteria are taken into account (case of companies with more than 250 employees)
-> 97/100 if the 4 criteria are taken into account (case of Sophysa in compliance with the legal obligations of the company).


Any questions about Sophysa, our products or career opportunities? Send us an e-mail, call us, or find a contact person at any of our locations worldwide.